SUMMARY: The US FDA on June 22, 2017, granted regular approval to the combination of RITUXAN® (Rituximab) and Hyaluronidase human (RITUXAN HYCELA®) for adult patients with Follicular Lymphoma, Diffuse Large B-Cell Lymphoma, and Chronic Lymphocytic Leukemia. RITUXAN HYCELA® is a combination of RITUXAN® and Hyaluronidase human (ENHANZE® technology), for SubCutaneous (SC) injection in multiple hematological malignancies. ENHANZE® is a drug delivery technology platform which removes limitations on the volume of biologics and drugs that can be delivered SubCutaneously, thereby significantly reducing the time required for drug administration.
RITUXAN® is a first generation type I, chimeric, monoclonal antibody that targets the CD20 antigen expressed on the surface of pre-B and mature B-lymphocytes. Upon binding to CD20, RITUXAN® mediates B-cell lysis. Possible mechanisms of cell lysis include Complement Dependent Cytotoxicity (CDC) and Antibody Dependent Cell mediated Cytotoxicity (ADCC).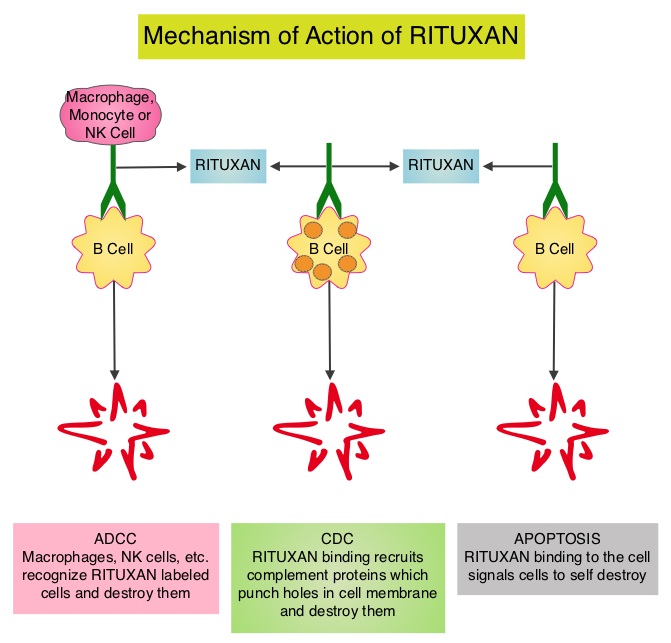 Hyaluronan is a polysaccharide present in the extracellular matrix of the subcutaneous tissue and is depolymerized by the naturally occurring enzyme Hyaluronidase. Hyaluronidase human increases permeability of the subcutaneous tissue locally for a period of 24-48 hrs, by temporarily depolymerizing Hyaluronan.
Hyaluronan is a polysaccharide present in the extracellular matrix of the subcutaneous tissue and is depolymerized by the naturally occurring enzyme Hyaluronidase. Hyaluronidase human increases permeability of the subcutaneous tissue locally for a period of 24-48 hrs, by temporarily depolymerizing Hyaluronan.
The approval of RITUXAN HYCELA® was based on several randomized clinical trials that demonstrated Non-inferior pharmacokinetics of SubCutaneous RITUXAN HYCELA® compared with IV RITUXAN® as well as comparable efficacy and safety results. The following are the specific trials included in the clinical development program:
MabEase phase III trial is an open label study which evaluated SubCutaneous (N=381) versus IV (N=195) RITUXAN® formulation plus CHOP chemotherapy, in treatment naïve patients with CD20-positive Diffuse Large B-Cell Lymphoma. There was no significant difference noted in the Objective Response Rate, Complete Response rates, Progression Free Survival and Overall Survival, between the two treatment groups.
SABRINA is a randomized phase III study that enrolled a total of 410 patients with previously untreated, CD20-positive Follicular Lymphoma and patients were randomized (1:1) to receive either a SC or IV RITUXAN® formulation in combination with CHOP or CVP chemotherapy. There was again no significant difference noted in the Objective Response Rate, Complete Response rates, Progression Free Survival and Overall Survival, between the two treatment groups
The SAWYER study is a phase Ib open label trial which compared the SC and IV formulations of RITUXAN® in combination with Fludarabine and Cyclophosphamide chemotherapy, in 176 treatment naïve patients with Chronic Lymphocytic Leukemia. There was no significant difference noted in the Objective Response Rate, Complete Response rates, Progression Free Survival and Overall Survival, between the two treatment groups.
SparkThera is a phase Ib study which investigated the pharmacokinetics and safety of SubCutaneous (SC) versus IV RITUXAN® formulations as maintenance therapy, in previously untreated or relapsed Follicular Lymphoma. It was noted that the pharmacokinetics of SC RITUXAN® formulation was Non-inferior to IV RITUXAN® formulation, with a comparable safety profile.
PrefMab is a randomized, open label, phase IIIb study which evaluated patient preference for SC or IV RITUXAN® formulation, in previously untreated CD20-positive Follicular lymphoma and Diffuse Large B-Cell Lymphoma. In this study, most patients preferred SC compared with IV formulation of RITUXAN®, mainly due to reduction in the duration and discomfort of administration.
Treatment with RITUXAN HYCELA® should be initiated only after patients had received at least one full dose RITUXAN® by intravenous infusion. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761064s000lbl.pdf

