SUMMARY: The FDA on October 19, 2016 granted accelerated approval to LARTRUVO® (Olaratumab) for the treatment of patients with Soft Tissue Sarcoma (STS), not amenable to curative treatment with radiotherapy or surgery, and with a histologic subtype for which an anthracycline containing regimen is appropriate. The American Cancer Society estimates that in 2016, about 12,310 new soft tissue sarcomas will be diagnosed in the United States and 4,990 patients will die of the disease. The most common types of Soft Tissue Sarcomas in adults are undifferentiated pleomorphic sarcoma (previously called Malignant Fibrous Histiocytoma), Liposarcoma, and Leiomyosarcoma. Patients with advanced Soft Tissue Sarcomas are often treated with a Doxorubicin based chemotherapy regimen and the median Overall Survival (OS) for those treated is 12-16 months.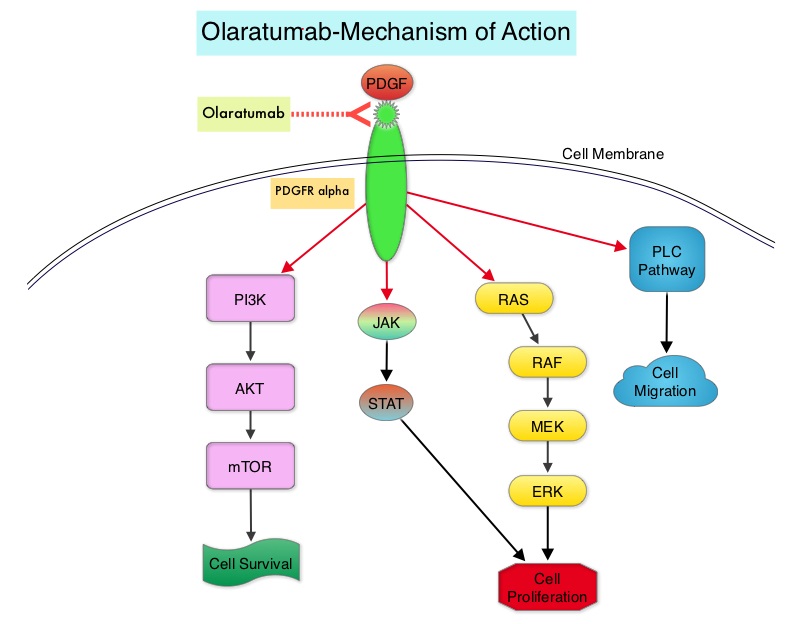
LARTRUVO® is a human IgG1 monoclonal antibody that binds to human PDGFRα with high affinity and blocks PDGFs (Platelet Derived Growth Factors) such as PDGF-AA, PDGF-BB, and PDGF-CC ligands, from binding to the receptor. Coexpression of PDGFRα and PDGFs, with associated autocrine-mediated cell growth, has been implicated in Sarcomas and Glioblastomas. Platelet Derived Growth Factor Receptor α (PDGFRα) is expressed in multiple tumor types and its aberrant activation may facilitate cancer development and spread.
The approval of LARTRUVO® was based on data from a randomized phase II study of Doxorubicin plus LARTRUVO® treatment, in patients with unresectable (locally advanced) or metastatic Soft Tissue Sarcoma (STS). In this pivotal trial, 133 patients with metastatic STS were randomized in a 1:1 ratio to receive LARTRUVO® plus Doxorubicin (N=66) or Doxorubicin alone (N=67). Enrolled patients had metastatic STS not amenable to curative treatment with surgery or radiotherapy, and a histologic type of sarcoma for which an anthracycline-containing regimen was appropriate, but had not been administered. LARTRUVO® was administered at 15 mg/kg as an IV infusion on days 1 and 8 of each 21-day cycle. All patients received doxorubicin 75 mg/m2 as an IV infusion on day 1 of each 21-day cycle for maximum of eight cycles. Single-agent LARTRUVO® was offered to patients in the Doxorubicin alone arm at the time of disease progression. The median patient age in the combination arm was 58.5 years and 88% of patients were positive for PDGFRα. Over a third of the enrolled patients had Leiomyosarcoma and over 25 different STS histologies were included in this study.
It was noted that there was a statistically significant improvement in Overall Survival (OS) for the combination treatment group with a median OS of 26.5 months compared to 14.7 months for those receiving Doxorubicin alone (HR=0.52; P<0.05). The median Progression Free Survival (independent review) was 8.2 months for patients in the combination group and 4.4 months for those receiving Doxorubicin alone (HR=0.74) and the Overall Response Rate (independent review) was 18% in the combination group and 8% in the Doxorubicin alone group. The most common (greater than or equal to 20%) side effects of treatment with LARTRUVO® were nausea, fatigue, neutropenia, musculoskeletal pain, mucositis, alopecia, vomiting, diarrhea, decreased appetite, abdominal pain, neuropathy, and headache. Infusion related reactions were seen in 13% of patients.
It was concluded that the combination of LARTRUVO® with Doxorubicin reduced the risk of death by 48% compared with Doxorubicin alone, for patients with advanced STS and is the first new therapy approved by the FDA for the initial treatment of Soft Tissue Sarcoma since the approval of Doxorubicin, more than 4 decades ago. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Tap WD, Jones RL, Van Tine BA, et al. Lancet. 2016;388:488-497

