SUMMARY: The FDA on November 23, 2015 approved OPDIVO® (Nivolumab) for the treatment of advanced Renal Cell Carcinoma in patients who have received prior anti-angiogenic therapy. The American Cancer Society estimates that about 61,560 new cases of kidney cancer will be diagnosed in the United States in 2015 and over 14,000 patients will die from this disease. The understanding of the Immune checkpoints has lead to the development of novel immunotherapies. Immune checkpoints or gate keepers are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells may be related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies have been developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as PD-1(Programmed cell Death-1), etc. Following inhibition of PD-1 by specific antibodies, T cells are unleashed, resulting in T cell proliferation and activation with subsequent therapeutic responses. OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. OPDIVO® in previously reported studies demonstrated an Objective Response Rate (ORR) of 20-22% and an Overall Survival (OS) of 18-25 months in previously treated patient with metastatic Renal Cell Carcinoma. AFINITOR® (Everolimus) is an inhibitor of mTOR (Mammalian Target of Rapamycin), which is a serine/threonine kinase. With the inhibition of mTOR, protein synthesis is down regulated resulting in decreased angiogenesis, cell proliferation and survival. AFINITOR® is presently indicated for the treatment of patients with advanced Renal Cell Carcinoma after failure, following treatment with SUTENT® (Sunitinib) or NEXAVAR® (Sorafenib) and has demonstrated improved median Progression Free Survival compared to placebo.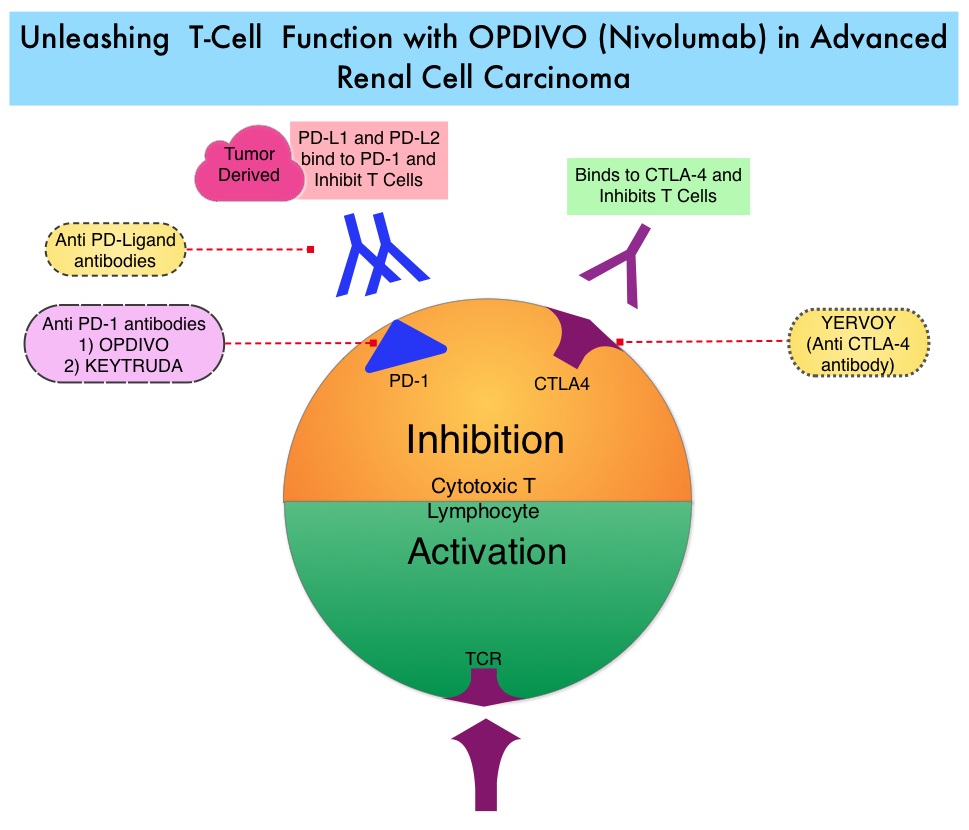
The FDA approval of OPDIVO® for Renal Cell Carcinoma (RCC), was based on a randomized, open-label, phase III study, in which 821 previously treated patients with advanced clear cell RCC, were randomly assigned in a 1:1 ratio to receive either OPDIVO® (N=410) or AFINITOR® (N=411). Treatment consisted of OPDIVO® 3 mg/kg IV every 2 weeks or AFINITOR® 10 mg PO daily. Enrolled patients had received prior treatment with one or two regimens of anti-angiogenic therapy which included SUTENT® (Sunitinib), VOTRIENT® (Pazopanib) and INLYTA® (Axitinib). The median age was 62 years. The primary end point was Overall Survival and secondary end points included Objective Response Rate and safety.
It was noted that the median Overall Survival in the OPDIVO® group was 25 months and 19.6 months in the AFINITOR® group, and this meant a 27% reduction in the risk of death with OPDIVO® (HR=0.73; P=0.002). This survival benefit was noted regardless of the PD-L1 expression level of the patient’s kidney tumors. The Objective Response Rate was greater with OPDIVO® compared with AFINITOR® (25% vs 5%; P<0.001) and the median Progression Free Survival was 4.6 months with OPDIVO® and 4.4 months with AFINITOR® (HR=0.88; P=0.11). Treatment related grade 3 or 4 adverse events occurred in 19% of the patients receiving OPDIVO® and in 37% of the patients receiving AFINITOR®.
The authors concluded that OPDIVO® significantly improved Overall Survival in patients with previously treated metastatic Renal Cell Carcinoma and is one of the few therapies with survival benefit, in this patient population. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. Motzer RJ, Escudier B, Dermott DF, et al. for the CheckMate 025 Investigators. N Engl J Med 2015; 373:1803-1813

