SUMMARY: The FDA on February 19, 2016, approved IBRANCE® (Palbociclib) in combination with FASLODEX® (Fulvestrant), for the treatment of women with Hormone Receptor (HR)-positive, Human Epidermal growth factor Receptor 2 (HER2)-negative advanced or metastatic breast cancer, with disease progression following endocrine therapy. Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 246,660 new cases of invasive breast cancer will be diagnosed in 2016 and over 40,450 women will die of the disease. Estrogen Receptor (ER) positive breast cancer cells are driven by estrogens. NOLVADEX® (Tamoxifen) is a nonsteroidal Selective Estrogen Receptor Modulator (SERM) and works mainly by binding to the Estrogen Receptor and thus blocks the proliferative actions of estrogen on the mammary tissue. ARIMIDEX® (Anastrozole) and FEMARA® (Letrozole) are nonsteroidal Aromatase Inhibitors that binds reversibly to the aromatase enzyme and inhibit the conversion of androgens to estrogens in the extra-gonadal tissues. Approximately 80% of breast tumors express Estrogen Receptors and/or Progesterone Receptors and these patients are often treated with anti-estrogen therapy as first line treatment.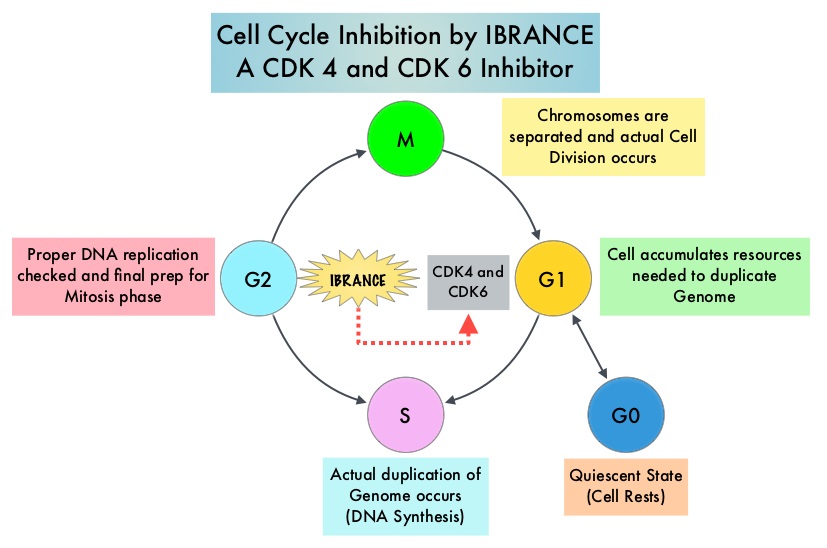
Cyclin Dependent Kinases (CDK) play a very important role to facilitate orderly and controlled progression of the cell cycle. Genetic alterations in these kinases and their regulatory proteins have been implicated in various malignancies. Cyclin Dependent Kinases 4 and 6 (CDK4 and CDK6), phosphorylate RetinoBlastoma protein (RB), and initiate transition from the G1 phase to the S phase of the cell cycle. CDK4 and CDK6 are activated in hormone receptor positive breast cancer, promoting breast cancer cell proliferation. Further, there is evidence to suggest that endocrine resistant breast cancer cell lines depend on CDK4 for cell proliferation. IBRANCE® (Palbociclib) is a reversible, oral, selective, small molecule inhibitor of Cyclin Dependent Kinases, CDK4 and CDK6, and prevents RB1 phosphorylation. IBRANCE® is the first CDK inhibitor approved by the FDA. It exhibits synergy when combined with endocrine therapies. In an open-label, randomized, phase II study, which included treatment naïve postmenopausal women with ER-positive, HER2-negative, advanced breast cancer, IBRANCE® given along with Aromatase Inhibitor FEMARA® (Letrozole), significantly prolonged Progression Free Survival, Overall Response rate and median duration of response, compared to FEMARA® alone. Based on this data, the U. S. Food and Drug Administration in February 2015, granted accelerated approval to IBRANCE® (Palbociclib), for use in combination with FEMARA®, in this patient population. FASLODEX® (Fulvestrant) is a selective estrogen receptor down-regulator presently indicated for the treatment of hormone receptor positive metastatic breast cancer patients, with disease progression following antiestrogen therapy.
The PALOMA3 is double-blind, phase 3 study in which the efficacy and safety of the combination of IBRANCE® and FASLODEX® was evaluated, in premenopausal or postmenopausal women, with hormone receptor positive, HER-2 negative, advanced breast cancer, who had disease progression during prior endocrine therapy. Five hundred and twenty one (N=521) patients were randomly assigned in a 2:1 ratio to receive either FASLODEX® 500 mg IM on days 1 and 15 during cycle 1, of a 28 day cycle, and then on day 1 of each cycle thereafter, along with IBRANCE® 125 mg PO daily for 3 weeks, followed by 1 week off (N=347) or FASLODEX® and placebo (N=174). ZOLADEX® (Goserelin) was administered to premenopausal or perimenopausal patients for the duration of study treatment, starting at least 4 weeks before randomization and continuing every 28 days. The median age was 57 years. One previous line of chemotherapy for metastatic disease was allowed and 79% were post-menopausal, 60% had visceral disease and 75% of the patients had received a previous chemotherapy regimen.
The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Overall Survival (OS), Response Rates, safety and tolerability. At the time of the preplanned interim analysis, the median Progression Free Survival was 9.2 months in the FASLODEX® / IBRANCE® group and 3.8 months in the FASLODEX® /placebo group (HR=0.42; P<0.001). This PFS benefit was observed across all prespecified patient subgroups, regardless of menopausal status. The most common grade 3 or 4 adverse events in the FASLODEX® / IBRANCE® group were neutropenia (62.0%, vs. 0.6%) and fatigue (2.0% vs. 1.2%). The incidence of febrile neutropenia was very rare (0.6%) and similar in both treatment groups. Treatment discontinuation rate due to adverse events was 2.6% in the IBRANCE® group and 1.7% in the placebo group.
The authors concluded that IBRANCE® in combination with FASLODEX® more than doubled the Progression Free Survival in advanced breast cancer patients, with hormone receptor positive and HER-2 negative disease, who had progressed on prior endocrine therapy. This study has reinforced the importance of CDK4 and CDK6, as key targets for hormone receptor positive breast cancer. Palbociclib in Hormone-Receptor–Positive Advanced Breast Cancer. Turner NC, Ro J, Andre F, et al. N Engl J Med 2015; 373:209-219

