SUMMARY: The FDA on April 24, 2015 approved CYRAMZA® for use in combination with FOLFIRI for the treatment of patients with metastatic ColoRectal Cancer (mCRC), whose disease has progressed on a first line AVASTIN® (Bevacizumab), ELOXATIN® (Oxaliplatin) and Fluoropyrimidine containing regimen. The American Cancer Society estimates that approximately 133,000 new cases of ColoRectal Cancer (CRC) will be diagnosed in the United States in 2015 and close to 50,000 are expected to die of the disease. With the availability of new active agents for the treatment of CRC, improving Overall Survival for patients with advanced disease, by prudently planning a treatment strategy and properly sequencing these agents, has become increasing relevant. 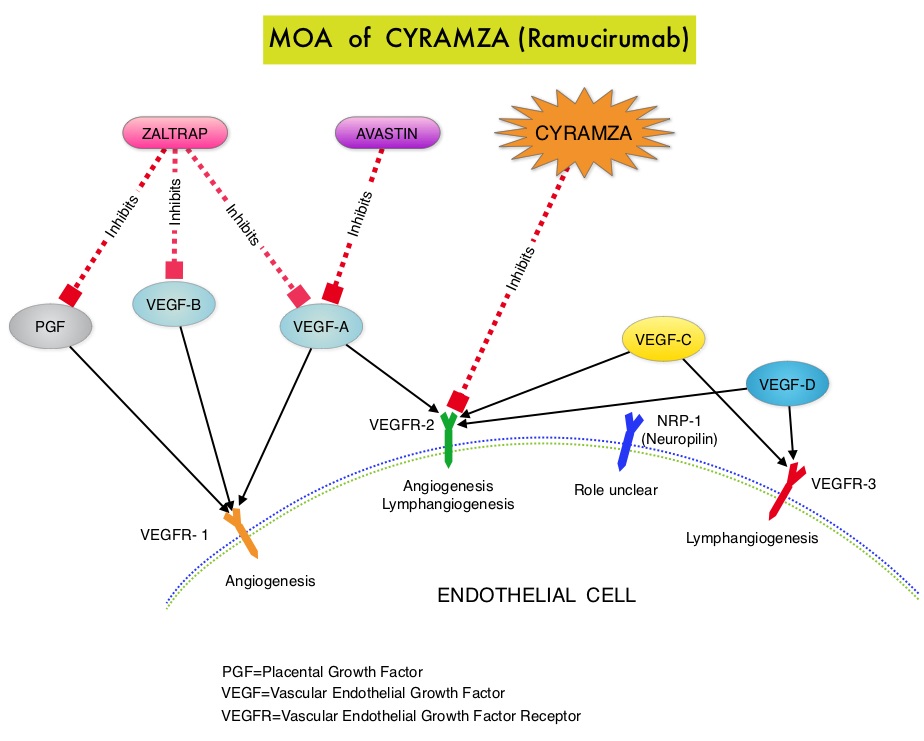 CYRAMZA® (Ramucirumab) is a recombinant human IgG1 monoclonal antibody that binds to Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2) and blocks binding of the VEGFR ligands VEGF-A, VEGF-C, and VEGF-D and thus inhibits ligand-induced proliferation and migration of endothelial cells. This is unlike AVASTIN®, which inhibits VEGF-A. Several studies have demonstrated that continuing VEGF inhibition along with standard second-line chemotherapy beyond disease progression has a significant advantage, in patients with mCRC. Two observational studies (BRiTE and ARIES) as well as an open label phase III study have all shown improvement in median Overall Survival (OS) when AVASTIN® was continued beyond first progression and given along with second line chemotherapy. In the VELOUR trial, FOLFIRI in combination with ZALTRAP® (Aflibercept), a VEGF-A, VEGF-B and Placental Growth Factor inhibitor, when given as second line therapy for those patients with mCRC who had progressed on AVASTIN® plus ELOXATIN® containing regimen, resulted in a significant improvement in the median PFS and OS.
CYRAMZA® (Ramucirumab) is a recombinant human IgG1 monoclonal antibody that binds to Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2) and blocks binding of the VEGFR ligands VEGF-A, VEGF-C, and VEGF-D and thus inhibits ligand-induced proliferation and migration of endothelial cells. This is unlike AVASTIN®, which inhibits VEGF-A. Several studies have demonstrated that continuing VEGF inhibition along with standard second-line chemotherapy beyond disease progression has a significant advantage, in patients with mCRC. Two observational studies (BRiTE and ARIES) as well as an open label phase III study have all shown improvement in median Overall Survival (OS) when AVASTIN® was continued beyond first progression and given along with second line chemotherapy. In the VELOUR trial, FOLFIRI in combination with ZALTRAP® (Aflibercept), a VEGF-A, VEGF-B and Placental Growth Factor inhibitor, when given as second line therapy for those patients with mCRC who had progressed on AVASTIN® plus ELOXATIN® containing regimen, resulted in a significant improvement in the median PFS and OS.
The RAISE trial is a double-blind, phase III study in which 1072 patients with mCRC who progressed on or after first-line treatment with AVASTIN®, ELOXATIN® and a Fluoropyrimidine were randomized to receive FOLFIRI given along with antiangiogenic agent CYRAMZA® (N = 536) or placebo (N = 536), every 2 weeks. The FOLFIRI regimen consisted of CAMPTOSAR® (Irinotecan) 180 mg/m2 IV, Folinic Acid 400 mg/m2 IV and 5-FU 400 mg/m2 IV bolus followed by 5-FU 2400 mg/m2 continuous IV infusion over 46 to 48 hours. CYRAMZA® was administered at a dose of 8 mg/kg IV every 2 weeks. The median age of patients was 62 years, about 50% harbored KRAS mutations and 99% of the patients had an ECOG performance status of 0 or 1. Treatment was continued until disease progression or unacceptable toxicity. The primary endpoint of the study was Overall Survival (OS) and secondary endpoints included Progression Free Survival (PFS), Objective Response Rate (ORR) and toxicity. There was a statistically significant Overall Survival improvement in patients receiving FOLFIRI plus CYRAMZA® compared to those receiving FOLFIRI plus placebo with a median OS of 13.3 and 11.7 months for patients on the FOLFIRI plus CYRAMZA® and FOLFIRI plus placebo arms, respectively (HR=0.85; P=0.023). The median PFS with FOLFIRI and CYRAMZA® was 5.7 months versus 4.5 months with FOLFIRI and placebo (HR= 0.79; P=0.001). There was no statistically significant difference in Objective Response Rate noted in the two treatment groups. Compared to the placebo group, more patients receiving CYRAMZA®, experienced grade 3 or more adverse events such as neutropenia (38.4% vs 23.3%) and hypertension (10.8% vs 2.8%). Other adverse events associated with CYRAMZA® included hemorrhage (43.9% vs 22.7% with placebo) and proteinuria (17% vs 4.5% with placebo). The authors concluded that CYRAMZA® is a new treatment option for second line treatment of patients with metastatic ColoRectal Cancer, including those with KRAS mutations. Tabernero J, Cohn AL, Obermannova R, et al. RAISE: A randomized, double-blind, multicenter phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab (RAM) or placebo (PBO) in patients (pts) with metastatic colorectal carcinoma (CRC) progressive during or following first-line combination therapy with bevacizumab (bev), oxaliplatin (ox), and a fluoropyrimidine (fp). 2015 Gastrointestinal Cancers Symposium; January 15-17, 2015; San Francisco, CA. Abstract 512.

