SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. It is the leading cause of cancer death among both men and women. The American Cancer Society estimates that over 224,000 new cases of lung cancer will be diagnosed in the United States in 2014 and over 159,000 will die of the disease. With changes in the cigarette composition and decline in tobacco consumption over the past several decades, adenocarcinoma now is the most frequent histologic subtype of lung cancer.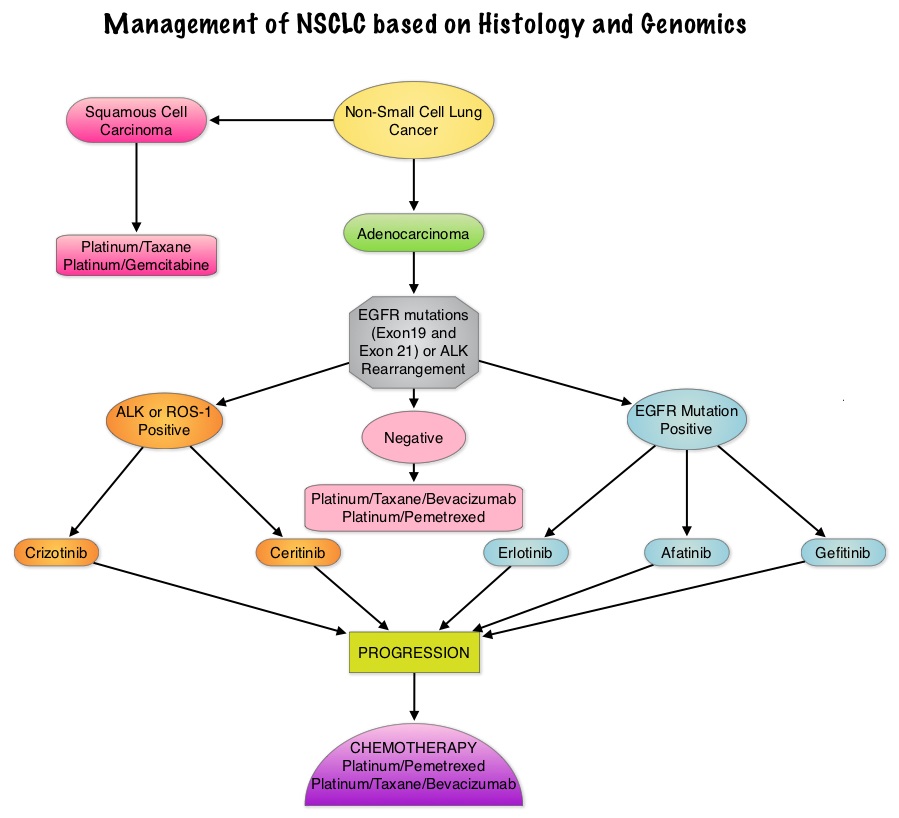 In 2004, the discovery of Epidermal Growth Factor Receptor (EGFR) mutations in some advanced Non Small Cell Lung Cancer (NSCLC) cases with adenocarcinoma histology and the favorable responses with EGFR Tyrosine Kinase Inhibitors (TKIs) such as TARCEVA® (Erlotinib) and IRESSA® (Gefitinib), changed the treatment paradigm in favor of targeted therapy, for this patient subset. It is estimated that approximately 10% of Western patient population and 50% of Asian patients with NSCLC, harbor EGFR activating mutations. EGFR Tyrosine Kinase Inhibitors have been shown to be superior to conventional chemotherapy in this patient group with improved Progression Free Survival (PFS) and Objective Response Rates. Patients with NSCLC should therefore be tested for the most common sensitizing mutations such as deletions in exon 19 and L858R point mutations in exon 21, as these patients clearly benefit from first line EGFR TKIs. EGFR expression by IHC (ImmunoHistoChemical) staining, EGFR gene copy by FISH (Fluorescence In Situ Hybridization) and blood based proteonomic testing by VERISTRAT® is currently not recommended for the selection of first line EGFR TKIs. There is presently no evidence indicating superiority of TKIs when compared with conventional chemotherapy for the second or third line treatment of EGFR Wild Type NSCLC. Nonetheless, TKIs are often recommended due to their acceptable toxicities. To address this treatment dilemma, the authors performed a systematic review and meta-analysis of randomized controlled trials, comparing first-generation EGFR TKIs (TARCEVA® and IRESSA®) treatment with conventional chemotherapy, in patients with advanced NSCLC, harboring Wild Type EGFR. This pooled analysis included 1605 patients from 11 clinical trials, with EGFR Wild Type NSCLC. The primary outcome measured was Progression Free Survival (PFS) and secondary outcomes were Objective Response Rate and Overall Survival. It was noted in this analysis that conventional chemotherapy was associated with longer PFS, compared with EGFR TKIs, among patients harboring Wild Type EGFR tumors. The authors noted that there was significant PFS benefit with chemotherapy, in trials using more sensitive EGFR mutation analysis platforms, than direct Sanger sequencing, and this may be the result of identifying the “true” Wild Type EGFR tumors. The objective response rate was higher at 16.8% with chemotherapy versus 7.2% for TKIs. There was however no statistically significant difference in the overall survival between the chemotherapy and TKI groups. When subgroups of patients in these trials were analyzed, outcomes were similar regardless of line of treatment, dominant ethinicity or EGFR mutation analysis method. The lack of improvement in Overall Survival in the chemotherapy group has been attributed to the large cross over rates in the trials that were analyzed. The authors concluded that conventional chemotherapy is associated with superior Progression Free Survival and Objective Response Rates, in patients with advanced NSCLC, harboring Wild Type EGFR tumors, compared with EGFR TKIs and the present guidelines recommending EGFR TKIs in this patient group has to be reevaluated. Lee J, Hahn S, Kim D, et al. JAMA 2014;311:1430-1437
In 2004, the discovery of Epidermal Growth Factor Receptor (EGFR) mutations in some advanced Non Small Cell Lung Cancer (NSCLC) cases with adenocarcinoma histology and the favorable responses with EGFR Tyrosine Kinase Inhibitors (TKIs) such as TARCEVA® (Erlotinib) and IRESSA® (Gefitinib), changed the treatment paradigm in favor of targeted therapy, for this patient subset. It is estimated that approximately 10% of Western patient population and 50% of Asian patients with NSCLC, harbor EGFR activating mutations. EGFR Tyrosine Kinase Inhibitors have been shown to be superior to conventional chemotherapy in this patient group with improved Progression Free Survival (PFS) and Objective Response Rates. Patients with NSCLC should therefore be tested for the most common sensitizing mutations such as deletions in exon 19 and L858R point mutations in exon 21, as these patients clearly benefit from first line EGFR TKIs. EGFR expression by IHC (ImmunoHistoChemical) staining, EGFR gene copy by FISH (Fluorescence In Situ Hybridization) and blood based proteonomic testing by VERISTRAT® is currently not recommended for the selection of first line EGFR TKIs. There is presently no evidence indicating superiority of TKIs when compared with conventional chemotherapy for the second or third line treatment of EGFR Wild Type NSCLC. Nonetheless, TKIs are often recommended due to their acceptable toxicities. To address this treatment dilemma, the authors performed a systematic review and meta-analysis of randomized controlled trials, comparing first-generation EGFR TKIs (TARCEVA® and IRESSA®) treatment with conventional chemotherapy, in patients with advanced NSCLC, harboring Wild Type EGFR. This pooled analysis included 1605 patients from 11 clinical trials, with EGFR Wild Type NSCLC. The primary outcome measured was Progression Free Survival (PFS) and secondary outcomes were Objective Response Rate and Overall Survival. It was noted in this analysis that conventional chemotherapy was associated with longer PFS, compared with EGFR TKIs, among patients harboring Wild Type EGFR tumors. The authors noted that there was significant PFS benefit with chemotherapy, in trials using more sensitive EGFR mutation analysis platforms, than direct Sanger sequencing, and this may be the result of identifying the “true” Wild Type EGFR tumors. The objective response rate was higher at 16.8% with chemotherapy versus 7.2% for TKIs. There was however no statistically significant difference in the overall survival between the chemotherapy and TKI groups. When subgroups of patients in these trials were analyzed, outcomes were similar regardless of line of treatment, dominant ethinicity or EGFR mutation analysis method. The lack of improvement in Overall Survival in the chemotherapy group has been attributed to the large cross over rates in the trials that were analyzed. The authors concluded that conventional chemotherapy is associated with superior Progression Free Survival and Objective Response Rates, in patients with advanced NSCLC, harboring Wild Type EGFR tumors, compared with EGFR TKIs and the present guidelines recommending EGFR TKIs in this patient group has to be reevaluated. Lee J, Hahn S, Kim D, et al. JAMA 2014;311:1430-1437

