SUMMARY: The American Cancer Society estimates that in 2015, close to 49,000 people will be diagnosed with Pancreatic Cancer in the United States and over 40,000 people will die of the disease. Some important risk factors for Pancreatic Cancer include increasing age, obesity, smoking history, genetic predisposition, exposure to certain dyes and chemicals, heavy alcohol use and pancreatitis. The best chance for long term survival is complete surgical resection, although this may not be feasible in a majority of the patients, as they present with advanced disease at the time of diagnosis. Based on the National Cancer Data Base, the 5 year observed survival rate for patients diagnosed with exocrine cancer of the pancreas is 14% for those with Stage IA disease and 1% for those with Stage IV disease. The FDA recently granted Breakthrough Therapy Designation status for the combination treatment that consists of two vaccines, GVAX and CRS-207, for patients with advanced Pancreatic Carcinoma. This designation was based on a phase II clinical trial in which the authors took a novel approach and tested a combination of two vaccines in patients with metastatic Pancreatic Adenocarcinoma. Traditional vaccination against specific bacterial and viral infections involves the injection of the specific weakened bacteria/virus or a structural component of the bacteria or virus. The body then mounts an immune response and is ready to respond to an infection associated with that specific bacteria or virus. Use of vaccines in cancer treatment is based on the same principle. 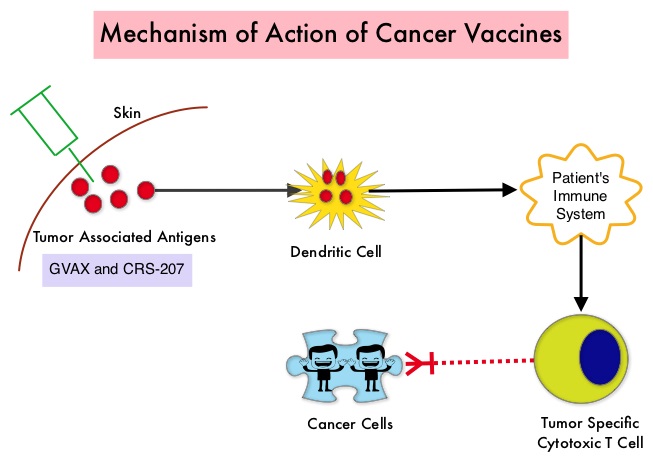 The two vaccines studied were GVAX and CRS-207. GVAX is an allogeneic whole cell vaccine developed from Pancreatic Cancer cell lines. These cancer cells are irradiated, to prevent them from dividing and are genetically modified to secrete GM-CSF (Granulocyte Macrophage Colony Stimulating Factor). GM-CSF is important for the growth and activation of dendritic cells also known as Antigen Presenting Cells. This vaccine when injected attracts the dendritic cells to the vaccine injection site and the dendritic cells in turn, pick up the antigens from the vaccine and present them to the patient’s immune system. The immune system then mounts a response by activating tumor specific T-cells. This vaccine therefore theoretically boosts the body’s immune system to fight the patient’s tumor, without causing collateral damage. The second vaccine CRS-207 is live-attenuated (weakened) Listeria monocytogenes bacterium which expresses mesothelin and stimulates innate and adaptive immunity. It is genetically engineered to elicit an immune response against the tumor-associated antigen mesothelin, which has been shown to be expressed at higher levels on Pancreatic Cancer cells than on normal cells. Previous studies have demonstrated that survival can be improved by induction of mesothelin specific T-cell responses. In this study, 90 patients with metastatic Pancreatic Adenocarcinoma were randomly assigned in a 2:1 ratio to receive two doses of GVAX followed by four doses of CRS-207 in Group A and six doses of GVAX alone in the Group B. Treatment was given every 3 weeks and low-dose CYTOXAN® (Cyclophosphamide) was given IV, the day before GVAX in both groups, to inhibit regulatory (suppressive) T-cells. More than 80% of the patients had at least one prior treatment for metastatic disease and 50% had two or more prior treatments. The Primary endpoint was overall survival. Secondary endpoints included safety, clinical and immune responses. At a planned interim analysis, the median Overall Survival (OS) was 6.1 months in Group A patients who had received the combination of two vaccines compared to 3.9 months in Group B patients who received GVAX alone (HR=0.59, P=0.02), resulting in a 41% reduction in risk of death with the combination immunotherapy. The one year survival probability doubled with the dual vaccine with an estimated one year survival of 24% for the combination immunotherapy group (Group A) compared with 12% for the GVAX alone group (Group B). The median OS in an updated analysis of patients who received three total doses which included at least two doses of GVAX and at least one dose of CRS-207 was 9.7 months compared to 4.6 months for those who received three doses of GVAX alone (HR=0.53, P=0.02), a 47% reduction in the risk of death. In the subgroup of patients who had two or more prior chemotherapy regimens, combination immunotherapy given as third line therapy or greater resulted in a median OS of 5.7 months compared to 3.7 months with GVAX alone (HR=0.30, P<0.001), a 70% reduction in risk of death. Stabilization or reduction of tumor marker CA 19-9, was seen in 27% of patients receiving combination immunotherapy compared to 9% in those who received GVAX alone (P=0.08). The median OS in patients with stable or better CA 19-9 response was 10.3 months compared with 4 months in those with CA 19-9 progression (HR=0.43, P=0.02). Toxicities included local reactions after GVAX and transient fevers, chills, and lymphopenia after CRS-207 administration. The authors concluded that immunotherapy with a combination of two vaccines improved Overall Survival with minimal toxicity, for patients with metastatic Pancreatic Carcinoma, who had failed prior therapies. Safety and Survival With GVAX Pancreas Prime and Listeria Monocytogenes–Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. Le DT, Wang-Gillam A, Picozzi V, et al. J Clin Oncol 2015; 33:1325-1333
The two vaccines studied were GVAX and CRS-207. GVAX is an allogeneic whole cell vaccine developed from Pancreatic Cancer cell lines. These cancer cells are irradiated, to prevent them from dividing and are genetically modified to secrete GM-CSF (Granulocyte Macrophage Colony Stimulating Factor). GM-CSF is important for the growth and activation of dendritic cells also known as Antigen Presenting Cells. This vaccine when injected attracts the dendritic cells to the vaccine injection site and the dendritic cells in turn, pick up the antigens from the vaccine and present them to the patient’s immune system. The immune system then mounts a response by activating tumor specific T-cells. This vaccine therefore theoretically boosts the body’s immune system to fight the patient’s tumor, without causing collateral damage. The second vaccine CRS-207 is live-attenuated (weakened) Listeria monocytogenes bacterium which expresses mesothelin and stimulates innate and adaptive immunity. It is genetically engineered to elicit an immune response against the tumor-associated antigen mesothelin, which has been shown to be expressed at higher levels on Pancreatic Cancer cells than on normal cells. Previous studies have demonstrated that survival can be improved by induction of mesothelin specific T-cell responses. In this study, 90 patients with metastatic Pancreatic Adenocarcinoma were randomly assigned in a 2:1 ratio to receive two doses of GVAX followed by four doses of CRS-207 in Group A and six doses of GVAX alone in the Group B. Treatment was given every 3 weeks and low-dose CYTOXAN® (Cyclophosphamide) was given IV, the day before GVAX in both groups, to inhibit regulatory (suppressive) T-cells. More than 80% of the patients had at least one prior treatment for metastatic disease and 50% had two or more prior treatments. The Primary endpoint was overall survival. Secondary endpoints included safety, clinical and immune responses. At a planned interim analysis, the median Overall Survival (OS) was 6.1 months in Group A patients who had received the combination of two vaccines compared to 3.9 months in Group B patients who received GVAX alone (HR=0.59, P=0.02), resulting in a 41% reduction in risk of death with the combination immunotherapy. The one year survival probability doubled with the dual vaccine with an estimated one year survival of 24% for the combination immunotherapy group (Group A) compared with 12% for the GVAX alone group (Group B). The median OS in an updated analysis of patients who received three total doses which included at least two doses of GVAX and at least one dose of CRS-207 was 9.7 months compared to 4.6 months for those who received three doses of GVAX alone (HR=0.53, P=0.02), a 47% reduction in the risk of death. In the subgroup of patients who had two or more prior chemotherapy regimens, combination immunotherapy given as third line therapy or greater resulted in a median OS of 5.7 months compared to 3.7 months with GVAX alone (HR=0.30, P<0.001), a 70% reduction in risk of death. Stabilization or reduction of tumor marker CA 19-9, was seen in 27% of patients receiving combination immunotherapy compared to 9% in those who received GVAX alone (P=0.08). The median OS in patients with stable or better CA 19-9 response was 10.3 months compared with 4 months in those with CA 19-9 progression (HR=0.43, P=0.02). Toxicities included local reactions after GVAX and transient fevers, chills, and lymphopenia after CRS-207 administration. The authors concluded that immunotherapy with a combination of two vaccines improved Overall Survival with minimal toxicity, for patients with metastatic Pancreatic Carcinoma, who had failed prior therapies. Safety and Survival With GVAX Pancreas Prime and Listeria Monocytogenes–Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. Le DT, Wang-Gillam A, Picozzi V, et al. J Clin Oncol 2015; 33:1325-1333

