SUMMARY: Circulating tumor cells (CTCs) are epithelial cells that are shed into the circulation from a primary or metastatic tumor. After being shed, CTCs can remain in the circulation or undergo apoptosis. 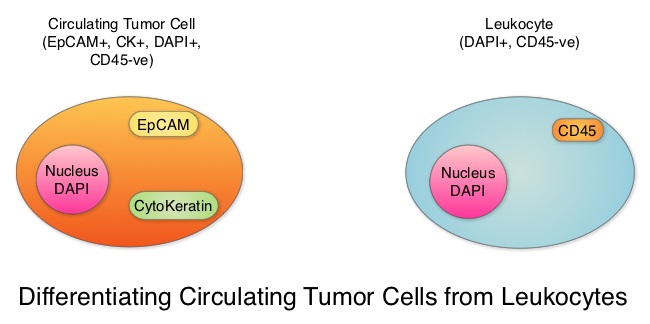 Evaluation of CTCs during the course of disease has prognostic value. Because of the very low concentrations of CTCs (1 CTC in the background of millions of normal hematopoietic cells) in the peripheral blood, different technologies have been developed that will allow enrichment and detection of these CTCs. One such technology is the CellSearch® system which is the first FDA-approved test for CTC assessment, in the peripheral blood of Metastatic Breast Cancer (MBC) patients. This automated system is able to enrich the peripheral blood sample with CTCs and the cells then are fluorescently stained for CytoKeratins (CK8,18 and 19), Common Leukocyte Antigen (CD45) and a nuclear dye (DAPI). CTCs are identified when they are CK positive, CD45 negative and DAPI positive. In essence, CTC assessment is a real time, peripheral blood evaluation (“Liquid Biopsy”) in MBC patients. The authors in this study conducted a pooled analysis to assess the clinical validity of CTCs in patients with MBC, as previously published studies reported contradictory results and were unable to ascertain whether enumeration of CTCs had better prognostic value than the traditional clinical and pathological features of the tumor and serum tumor markers. Data was gathered on 1944 patients with MBC, who had participated in clinical trials at 17 centers between 2003 and 2012. Participants in these studies were starting a new line of therapy (predominantly chemotherapy), and these studies had CTC quantification using CellSearch® platform, before start of new treatment (baseline), data for Progression Free Survival, Overall Survival or both. Using accepted statistical methodologies, the authors noted the following findings-
Evaluation of CTCs during the course of disease has prognostic value. Because of the very low concentrations of CTCs (1 CTC in the background of millions of normal hematopoietic cells) in the peripheral blood, different technologies have been developed that will allow enrichment and detection of these CTCs. One such technology is the CellSearch® system which is the first FDA-approved test for CTC assessment, in the peripheral blood of Metastatic Breast Cancer (MBC) patients. This automated system is able to enrich the peripheral blood sample with CTCs and the cells then are fluorescently stained for CytoKeratins (CK8,18 and 19), Common Leukocyte Antigen (CD45) and a nuclear dye (DAPI). CTCs are identified when they are CK positive, CD45 negative and DAPI positive. In essence, CTC assessment is a real time, peripheral blood evaluation (“Liquid Biopsy”) in MBC patients. The authors in this study conducted a pooled analysis to assess the clinical validity of CTCs in patients with MBC, as previously published studies reported contradictory results and were unable to ascertain whether enumeration of CTCs had better prognostic value than the traditional clinical and pathological features of the tumor and serum tumor markers. Data was gathered on 1944 patients with MBC, who had participated in clinical trials at 17 centers between 2003 and 2012. Participants in these studies were starting a new line of therapy (predominantly chemotherapy), and these studies had CTC quantification using CellSearch® platform, before start of new treatment (baseline), data for Progression Free Survival, Overall Survival or both. Using accepted statistical methodologies, the authors noted the following findings-
1) At baseline prior to starting treatment, 47% (N=911) patients had 5 or more CTCs per 7•5 mL of peripheral blood, suggesting more aggressive disease and this was associated with decreased Progression Free Survival (P<0•0001) and Overall Survival (P<0•0001), compared with patients with less than 5 CTCs per 7•5 mL at baseline.
2) At 3-5 weeks after start of treatment (1-2 cycles of treatment), when adjusted for baseline CTC count, 5 or more CTCs per 7•5 mL of peripheral blood was associated with shortened Progression Free Survival (P<0•0001) and Overall Survival (P<0•0001), suggesting that these patients were treatment resistant.
3) An early decrease in the CTC at week 3-5, from a high baseline of 5 or more CTCs per 7•5 mL to less than 5 CTCs per 7.5 ml was associated with significantly longer Progression Free Survival and Overall Survival.
4) Enumeration of CTCs was a better predictor of prognosis than mucin based serum biomarkers such as CEA and CA15-3.
The prognostic value of high CTC count at baseline and at 3-5 weeks of treatment, on Progression Free Survival and Overall Survival was maintained in all subgroups tested, regardless of breast cancer subtypes and type of therapy patients received. Based on this pooled analysis, with the largest assessment of CTC enumeration in MBC patients, the authors concluded that CTC count can prognosticate Progression Free Survival and Overall Survival early in the treatment course, allowing customized care. Further, CTC enumeration, unlike serum tumor markers, correlates with clinical and pathological characteristics. Bidard F, Peeters DJ, Fehm T, et al. The Lancet Oncology 2014;15:406-414

