SUMMARY: Circulating Tumor Cells (CTCs) are epithelial cells that are shed into the circulation from a primary or metastatic tumor. After being shed, CTCs can remain in the circulation or undergo apoptosis. Evaluation of CTCs during the course of disease has prognostic value. Because of the very low concentrations of CTCs (1 CTC in the background of millions of normal hematopoietic cells) in the peripheral blood, different technologies have been developed that will allow enrichment and detection of these CTCs. One such technology is the CellSearch® system which is the first FDA-approved test for CTC assessment, in the peripheral blood of patients with breast cancer. This automated system is able to enrich the peripheral blood sample with CTCs and the cells then are fluorescently stained for CytoKeratins (CK8,18 and 19), Common Leukocyte Antigen (CD45) and a nuclear dye (DAPI). CTCs are identified when they are CK positive, CD45 negative and DAPI positive. In essence, CTC assessment is a real time, peripheral blood evaluation (“Liquid Biopsy”) in breast cancer patients.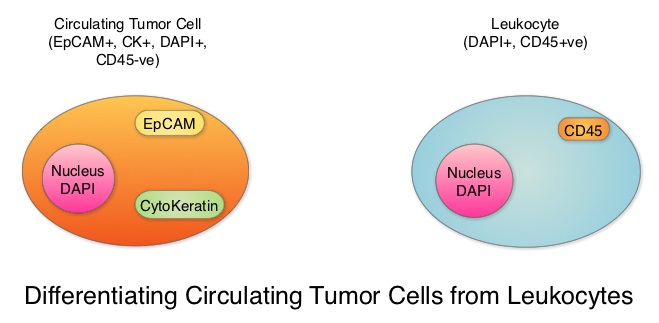
Despite advances in treatment, approximately 30% of patients with node-negative breast cancer and 50% of patients with node-positive breast cancer may relapse within 5 years. This is due to cancer cells shed from primary tumors that migrate to distal sites as Circulating Tumor Cells (CTCs) via the circulatory system. CTCs are therefore precursors of metastatic disease and may not only predict risk of metastatic disease but may also be useful in monitoring treatment efficacy. There have been conflicting reports about the effectiveness of different treatments to reduce CTCs in breast cancer patients, including the different molecular subtypes.
To further address these controversies, the authors conducted a meta-analysis of the published studies which included measurement of CTCs before and after treatment in breast cancer patients, and estimated the benefit of reducing CTC on patient outcomes. Data base searches included 1004 publications and 50 studies conducted between 2009 and 2016 in the US, Europe and Asia. A total of 6712 patients from these studies were eligible for meta-analysis. Enrolled patients had pathologically diagnosed breast cancer, CTCs were detected by any method, including cell capture and quantitative PCR and the patient’s CTC status both pre- and post-therapy was available. The CTC-positive rate was reported using different cut-off values of CTC count and different expression thresholds of epithelial genes (EpCAM, CK18, CK19) using RT-PCR in the various studies.
An overall analysis of the 6712 patients with CTC-positive rate by the random-effects model suggested that treatment intervention significantly decreased CTC-positive rate compared to the baseline (Relative Risk (RR)=0.68, P<0.00001, which meant a 32% reduction in the Relative Risk, compared to baseline values.
Subgroup analyses revealed that when compared to pre-treatment, CTCs were decreased after neoadjuvant treatment (RR=0.65, P=0.006), adjuvant treatment (RR=0.89, P=0.10), treatment in metastatic setting (RR=0.59, P<0.00001) and the combination therapy (RR=0.78, P=0.03). Reduction in CTCs was not seen after surgery (RR=1.27, P=0.42), suggesting that local intervention with surgery does not eliminate CTCs and patients with positive CTCs should receive other therapies after surgery, to decrease the risk of recurrence.
When compared to pre-treatment levels, treatment resulted in significant reduction in CTCs in HER2-positive patients (RR=0.68, P<0.0001) and HER2-negative patients (RR=0.52, P=0.01). This reduction in CTCs was however not noted in patients with triple-negative breast cancer patients (RR=0.38, P=0.29), indicating that current therapies for this group is inadequate and should be further optimized with newer therapies.
More importantly, reduction in CTCs was associated with lower probability of disease progression (P=0.01), longer Progression Free Survival (P<0.0001) and longer Overall Survival (P<0.00001).
It was concluded that based on this large meta-analysis, CTCs can help monitor the effectiveness of treatment and guide subsequent therapies in breast cancer patients. Circulating tumor cell status monitors the treatment responses in breast cancer patients: a meta-analysis. Yan W-T, Cui X, Chen Q, et al. Sci. Rep. 7, 43464; doi: 10.1038/srep43464 (2017).

