SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 246,660 new cases of invasive breast cancer will be diagnosed in 2016 and 40,450 women will die of the disease. Estrogen Receptor (ER) positive breast cancer cells are driven by estrogens. Approximately 80% of breast tumors express Estrogen Receptors and/or Progesterone Receptors and these patients are often treated with anti-estrogen therapy as first line treatment. Cyclin Dependent Kinases (CDK) play a very important role to facilitate orderly and controlled progression of the cell cycle. Genetic alterations in these kinases and their regulatory proteins have been implicated in various malignancies. Cyclin Dependent Kinases 4 and 6 (CDK4 and CDK6), phosphorylate RetinoBlastoma protein (RB), and initiate transition from the G1 phase to the S phase of the cell cycle. CDK4 and CDK6 are activated in hormone receptor positive breast cancer, promoting breast cancer cell proliferation. Further, there is evidence to suggest that endocrine resistant breast cancer cell lines depend on CDK4 for cell proliferation.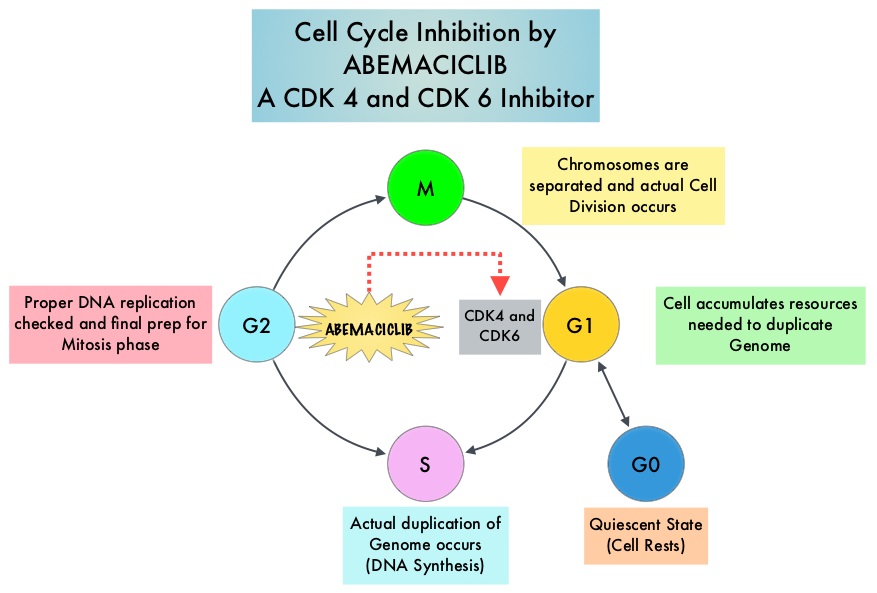
Abemaciclib is an oral, selective inhibitor of CDK4 and CDK6 kinase activity that prevents the phosphorylation and subsequent inactivation of the Rb tumor suppressor protein, thereby inducing G1 cell cycle arrest and inhibition of cell proliferation. The FDA granted breakthrough designation for Abemaciclib based on a phase I trial in which this agent demonstrated significant single agent activity in refractory Hormone Receptor (HR) positive metastatic breast cancer. MONARCH 1 is a single arm phase II study which evaluated the single-agent activity of Abemaciclib in heavily pretreated patients with HR-positive, HER2-negative metastatic breast cancer. This trial included 132 patients with HR-positive, HER2-negative metastatic breast cancer, whose disease progressed on or after endocrine therapy and chemotherapy. Patients had received a median of 3 lines of prior therapy for advanced disease and this included a median of 2 lines of chemotherapy. Approximately 50% of the patients had received FASLODEX® (Fulvestrant), 70% of patients had received Taxane based chemotherapy and 55 % of patients had received XELODA® (Capecitabine), in the metastatic setting. Abemaciclib was administered at 200 mg PO daily on a continuous schedule every 12 hours until disease progression. The median age was 58 yrs, 90% of the patients had visceral disease and 85% had at least 2 sites of metastatic disease. The primary endpoint was Objective Response Rate (ORR) and secondary endpoints included Duration of Response, Progression Free Survival (PFS), Overall Survival (OS), Clinical Benefit Rate (Complete Response plus Partial Response plus Stable Disease) and safety.
An interim analysis was performed at 8 months by when 35.6% of patients had received at least 8 cycles of Abemaciclib. The ORR was 17.4%, the Clinical Benefit Rate lasting for 6 months or more was 42.4%. The median time to response was 3.7 months and the median Duration of Response was 8.6 months. The median PFS was 5.7 months. The most common adverse events were diarrhea, fatigue, nausea, decreased appetite, abdominal pain and treatment discontinuation rate was infrequent at 6.8%.
It was concluded that CDK4 and CDK6 inhibitor, Abemaciclib, has significant antitumor activity in patients with refractory, HR-positive, HER2-negative metastatic breast cancer, for whom chemotherapy is the only option. Studies are underway combining Abemaciclib with FASLODEX®, for postmenopausal patients with HR-positive, HER2-negative, locally advanced or metastatic breast cancer who had progressed on 1 prior endocrine therapy (MONARCH-2 trial), as well as combining Abemaciclib with a nonsteroidal Aromatase Inhibitor in an earlier setting (MONARCH-3 trial), for patients with HR-positive, HER2-negative, locoregionally recurrent or metastatic breast cancer. MONARCH 1: Results from a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2- breast cancer, after chemotherapy for advanced disease. Dickler MN, Tolaney SM, Rugo HS, et al. J Clin Oncol 34, 2016 (suppl; abstr 510)

