SUMMARY:The FDA recently approved AVASTIN® (Bevacizumab) in combination with chemotherapy for the treatment of patients with platinum-resistant, recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer. It is estimated that in the United States, approximately 22,000 women will be diagnosed with ovarian cancer in 2014 and a little over 14,000 women will die of the disease. In spite of significantly improved median survival following aggressive surgical debulking and platinum plus taxane based therapy, long term cure rate is approximately 20-30%. Majority of the patients relapse in 18-24 months and 25% of these patients are Platinum Resistant. These platinum resistant patients are usually treated with single agent chemotherapy drugs such as DOXIL® (Pegylated Liposomal Doxorubicin-PLD), TAXOL® (Paclitaxel) and HYCAMTIN® (Topotecan), with an expected response rate of 10-15%, median response duration of about 3-4 months and median Overall Survival of approximately 12 months. AURELIA (Avastin Use in Platinum-Resistant Epithelial Ovarian Cancer) is a multicenter, randomized, open-label, Phase III study in which 361 women with platinum resistant recurrent epithelial ovarian, primary peritoneal or fallopian tube cancer were enrolled. These patients had disease progression within six months of their platinum based chemotherapy (Platinum Resistant) and were randomly assigned to receive AVASTIN® (Bevacizumab) 10 mg/kg every 2 weeks or 15 mg/kg every 3 weeks in combination with investigators choice of single agent chemotherapy agent (N=179) or single agent chemotherapy alone (N=182). Chemotherapy included one of the following agents – TAXOL® 80 mg/m2 on days 1, 8, 15 and 22 every 4 weeks, DOXIL® 40 mg/m2 on day 1 every 4 weeks or HYCAMTIN® either 4 mg/m2 on days 1, 8 and 15 every 4 weeks or 1.25 mg/m2 on days 1-5 every 3 weeks. Patients with refractory disease, history of bowel obstruction, or those who had received two or more prior anticancer regimens were excluded. Treatment was given until disease progression. Patients who had progressed on single agent chemotherapy were allowed to cross over to AVASTIN® group. 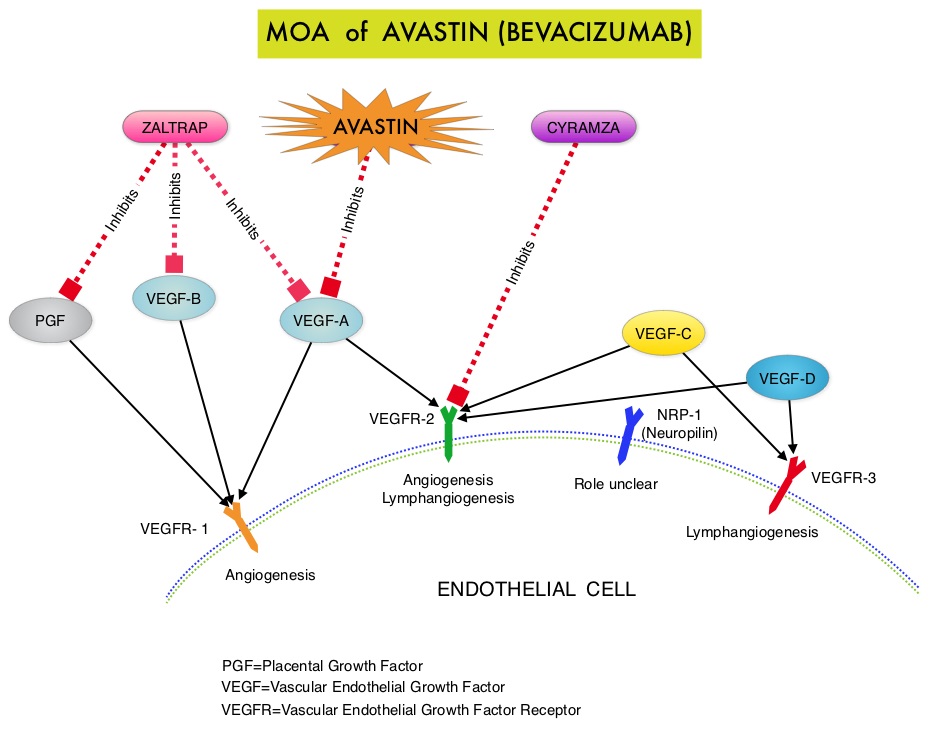 The primary end point was Progression Free Survival (PFS) and secondary end points included Objective Response Rate (ORR), Overall Survival (OS), safety, and patient reported outcomes. The combination of AVASTIN® plus chemotherapy resulted in a 62% reduction in the risk of progression compared to those who received chemotherapy alone, with a median PFS of 6.8 months for the AVASTIN® plus chemotherapy group versus 3.4 months for the single agent chemotherapy group (HR=0.38, P<0.0001) and thus met the primary endpoint of this clinical trial. This PFS benefit was seen consistently across all subgroups including the subgroup of patients with ascites. The ORR was 27.3% with the AVASTIN® combination versus 11.8% with single agent chemotherapy (P =0.001). The median OS was 16.6 months for the AVASTIN® combination versus 13.3 months for the single agent chemotherapy group (HR=0.85; P < .17). The lack of statistical significance in the OS has been attributed to cross over of 40% of patients, initially randomized to the chemotherapy alone group, who upon progression received AVASTIN®. There was a 15% improvement in abdominal and GI symptoms as reported by patients, with the AVASTIN® combination, compared to chemotherapy alone. On exploratory analyses it was noted that the addition of AVASTIN® to TAXOL® resulted in the most benefit, with a 5.7 month improvement in median PFS (9.6 versus 3.9 months), a 23% improvement in the overall response rate (53% versus 30%) and a 9.2 month improvement in median OS (22.4 versus 13.2 months) compared to single agent TAXOL®. This benefit was seen in spite of the fact that 97% of the patients in the TAXOL® group had received this agent with previous chemotherapy regimens. These findings suggest that patients who have received prior treatment with TAXOL® may benefit from AVASTIN® plus weekly TAXOL®. The most common adverse reactions (greater than or equal to 15%) in patients treated with AVASTIN® plus chemotherapy were neutropenia, peripheral neuropathy, hypertension and GI perforation occurred in 1.7% of these patients. This low perforation rate has been attributed to the exclusion of patients with rectosigmoid involvement by pelvic examination or bowel involvement on CT scan as well as those with clinical symptoms of bowel obstruction. The authors concluded that AVASTIN® in combination with chemotherapy significantly improved Progression Free Survival and Objective Response Rates in patients with Platinum Resistant Recurrent Ovarian Cancer. Pujade-Lauraine E, Hilpert F, Weber B, et al. J Clin Oncol 2014;32:1302-1308
The primary end point was Progression Free Survival (PFS) and secondary end points included Objective Response Rate (ORR), Overall Survival (OS), safety, and patient reported outcomes. The combination of AVASTIN® plus chemotherapy resulted in a 62% reduction in the risk of progression compared to those who received chemotherapy alone, with a median PFS of 6.8 months for the AVASTIN® plus chemotherapy group versus 3.4 months for the single agent chemotherapy group (HR=0.38, P<0.0001) and thus met the primary endpoint of this clinical trial. This PFS benefit was seen consistently across all subgroups including the subgroup of patients with ascites. The ORR was 27.3% with the AVASTIN® combination versus 11.8% with single agent chemotherapy (P =0.001). The median OS was 16.6 months for the AVASTIN® combination versus 13.3 months for the single agent chemotherapy group (HR=0.85; P < .17). The lack of statistical significance in the OS has been attributed to cross over of 40% of patients, initially randomized to the chemotherapy alone group, who upon progression received AVASTIN®. There was a 15% improvement in abdominal and GI symptoms as reported by patients, with the AVASTIN® combination, compared to chemotherapy alone. On exploratory analyses it was noted that the addition of AVASTIN® to TAXOL® resulted in the most benefit, with a 5.7 month improvement in median PFS (9.6 versus 3.9 months), a 23% improvement in the overall response rate (53% versus 30%) and a 9.2 month improvement in median OS (22.4 versus 13.2 months) compared to single agent TAXOL®. This benefit was seen in spite of the fact that 97% of the patients in the TAXOL® group had received this agent with previous chemotherapy regimens. These findings suggest that patients who have received prior treatment with TAXOL® may benefit from AVASTIN® plus weekly TAXOL®. The most common adverse reactions (greater than or equal to 15%) in patients treated with AVASTIN® plus chemotherapy were neutropenia, peripheral neuropathy, hypertension and GI perforation occurred in 1.7% of these patients. This low perforation rate has been attributed to the exclusion of patients with rectosigmoid involvement by pelvic examination or bowel involvement on CT scan as well as those with clinical symptoms of bowel obstruction. The authors concluded that AVASTIN® in combination with chemotherapy significantly improved Progression Free Survival and Objective Response Rates in patients with Platinum Resistant Recurrent Ovarian Cancer. Pujade-Lauraine E, Hilpert F, Weber B, et al. J Clin Oncol 2014;32:1302-1308
Bevacizumab Combined With Chemotherapy for Platinum-Resistant Recurrent Ovarian Cancer The AURELIA Open-Label Randomized Phase III Trial
SUMMARY: It is estimated that in the United States, approximately 22,000 women will be diagnosed with ovarian cancer in 2014 and a little over 14,000 women will die of the disease. In spite of significantly improved median survival following aggressive surgical debulking and platinum plus taxane based therapy, long term cure rate is approximately 20-30%. Majority of the patients relapse in 18-24 months and 25% of these patients are Platinum Resistant. These platinum resistant patients are usually treated with single agent chemotherapy drugs such as DOXIL® (Pegylated Liposomal Doxorubicin-PLD), TAXOL® (Paclitaxel) and HYCAMTIN® (Topotecan), with an expected response rate of 10-15%, median response duration of about 3-4 months and median Overall Survival of approximately 12 months.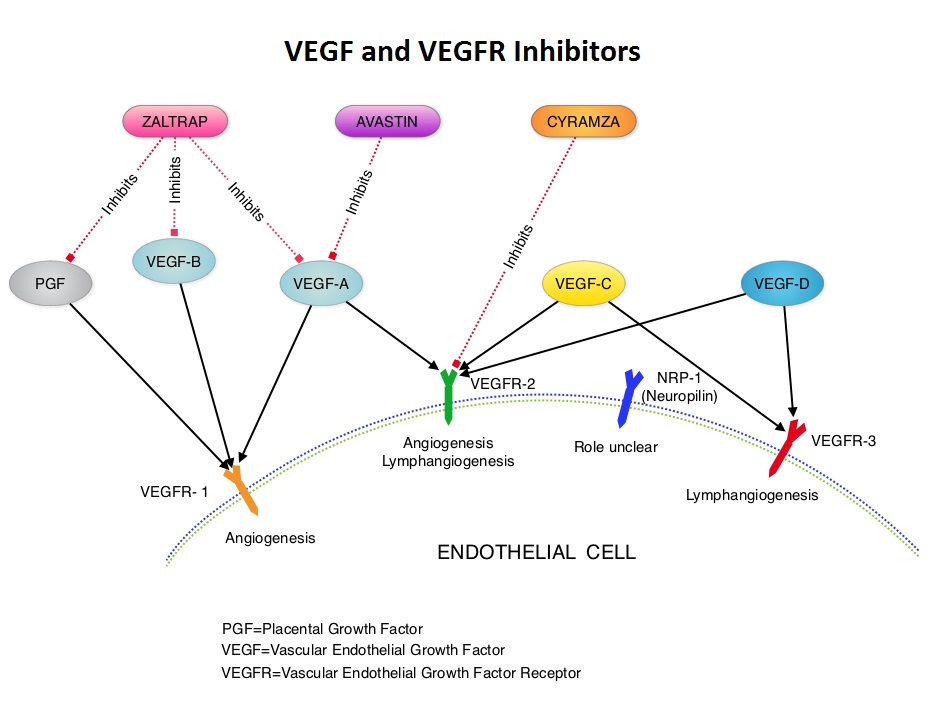 AURELIA (Avastin Use in Platinum-Resistant Epithelial Ovarian Cancer) is a multicenter, randomized, open-label, Phase III study in which 361 women with platinum resistant recurrent epithelial ovarian, primary peritoneal or fallopian tube cancer were enrolled. These patients had disease progression within six months of their platinum based chemotherapy (Platinum Resistant) and were randomly assigned to receive AVASTIN® (Bevacizumab) 10 mg/kg every 2 weeks or 15 mg/kg every 3 weeks in combination with investigators choice of single agent chemotherapy agent such as weekly TAXOL®, HYCAMTIN®, DOXIL® (N=179) or single agent chemotherapy alone (N=182). Patients with refractory disease, history of bowel obstruction, or those who had received two or more prior anticancer regimens were excluded. Treatment was given until disease progression. Patients who had progressed on single agent chemotherapy were allowed to cross over to single agent AVASTIN®. The primary end point was Progression Free Survival (PFS) and secondary end points included Objective Response Rate (ORR), Overall Survival (OS), safety, and patient reported outcomes. The combination of AVASTIN® plus chemotherapy resulted in a 52% reduction in the risk of progression compared to those who received chemotherapy alone, with a median PFS of 6.7 months for the AVASTIN® plus chemotherapy group vs 3.4 months for the single agent chemotherapy group (HR=0.48, P<0.001) and thus met the primary endpoint of this clinical trial. This PFS benefit was seen consistently across all subgroups including the subgroup of patients with ascites. The ORR was 27.3% with the AVASTIN® combination vs 11.8% with single agent chemotherapy (P =0.001). The median OS was 16.6 months for the AVASTIN® combination vs 13.3 months for the single agent chemotherapy group (HR=0.85; P < .17). The lack of statistical significance in the OS has been attributed to cross over of 40% of patients, initially randomized to the chemotherapy alone group, who upon progression, received single agent AVASTIN®. As expected, grade 2 or more hypertension and proteinuria were common in the AVASTIN® group and GI perforation occurred in 2.2% of these patients. There was a 15% improvement in abdominal and GI symptoms as reported by patients, with the AVASTIN® combination, compared to chemotherapy alone. The authors concluded that AVASTIN® in combination with chemotherapy significantly improved Progression Free Survival and Objective Response Rates in patients with Platinum Resistant recurrent Ovarian Cancer. Pujade-Lauraine E, Hilpert F, Weber B, et al. J Clin Oncol 2014;32:1302-1308
AURELIA (Avastin Use in Platinum-Resistant Epithelial Ovarian Cancer) is a multicenter, randomized, open-label, Phase III study in which 361 women with platinum resistant recurrent epithelial ovarian, primary peritoneal or fallopian tube cancer were enrolled. These patients had disease progression within six months of their platinum based chemotherapy (Platinum Resistant) and were randomly assigned to receive AVASTIN® (Bevacizumab) 10 mg/kg every 2 weeks or 15 mg/kg every 3 weeks in combination with investigators choice of single agent chemotherapy agent such as weekly TAXOL®, HYCAMTIN®, DOXIL® (N=179) or single agent chemotherapy alone (N=182). Patients with refractory disease, history of bowel obstruction, or those who had received two or more prior anticancer regimens were excluded. Treatment was given until disease progression. Patients who had progressed on single agent chemotherapy were allowed to cross over to single agent AVASTIN®. The primary end point was Progression Free Survival (PFS) and secondary end points included Objective Response Rate (ORR), Overall Survival (OS), safety, and patient reported outcomes. The combination of AVASTIN® plus chemotherapy resulted in a 52% reduction in the risk of progression compared to those who received chemotherapy alone, with a median PFS of 6.7 months for the AVASTIN® plus chemotherapy group vs 3.4 months for the single agent chemotherapy group (HR=0.48, P<0.001) and thus met the primary endpoint of this clinical trial. This PFS benefit was seen consistently across all subgroups including the subgroup of patients with ascites. The ORR was 27.3% with the AVASTIN® combination vs 11.8% with single agent chemotherapy (P =0.001). The median OS was 16.6 months for the AVASTIN® combination vs 13.3 months for the single agent chemotherapy group (HR=0.85; P < .17). The lack of statistical significance in the OS has been attributed to cross over of 40% of patients, initially randomized to the chemotherapy alone group, who upon progression, received single agent AVASTIN®. As expected, grade 2 or more hypertension and proteinuria were common in the AVASTIN® group and GI perforation occurred in 2.2% of these patients. There was a 15% improvement in abdominal and GI symptoms as reported by patients, with the AVASTIN® combination, compared to chemotherapy alone. The authors concluded that AVASTIN® in combination with chemotherapy significantly improved Progression Free Survival and Objective Response Rates in patients with Platinum Resistant recurrent Ovarian Cancer. Pujade-Lauraine E, Hilpert F, Weber B, et al. J Clin Oncol 2014;32:1302-1308

