SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. About 268,600 new cases of female breast cancer will be diagnosed in 2019 and about 41,760 women will die of the disease. Approximately 75% of patients with breast cancer are hormone receptor positive (Estrogen Receptor/Progesterone Receptor positive) and this is a predictor of response to endocrine therapy. These patients are often treated with anti-estrogen therapy as first line treatment. In premenopausal woman, the ovary is the main source of estrogen production, whereas in postmenopausal women, the primary source of estrogen is the Aromatase enzyme mediated conversion of androstenedione and testosterone to estrone and estradiol in extragonadal/peripheral tissues. In postmenopausal women with hormone receptor-positive, early-stage breast cancer, treatment with adjuvant Aromatase Inhibitors is the standard of care.
ARIMIDEX® (Anastrozole), FEMARA® (Letrozole) and AROMASIN® (Exemestane) are Aromatase Inhibitors that bind reversibly to the Aromatase enzyme and inhibit the conversion of androgens to estrogens in the extra-gonadal tissues. Aromatase Inhibitors however are associated with accelerated bone loss, leading to a decrease in Bone Mineral Density (BMD) and can thus cause osteopenia and osteoporosis, thereby increasing fracture risk.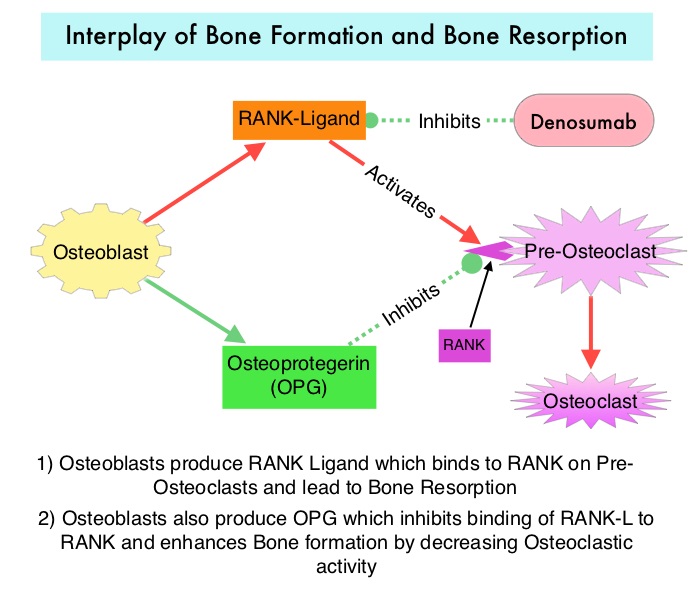
PROLIA® (Denosumab) is a monoclonal antibody that inhibits osteoclast formation, function and survival, by selectively targeting the RANK ligand. The RANK-RANK ligand system is an important mediator of signaling in the genesis of osteoclasts and bone resorption. Additionally, the RANK-RANK ligand system has been implicated in antitumor immunity and may have a role in suppressing tumor agenesis. It has been hypothesized that targeting RANK with anti-RANK inhibitor might reverse the immunosuppressive effect of the RANK-RANK ligand signaling pathway.
ABCSG-18 is a randomized, double-blind, placebo controlled, Phase III trial in which the authors evaluated the benefits of the anti-RANK ligand antibody PROLIA® on bone health, in postmenopausal patients, with early stage hormone receptor-positive breast cancer, treated with Aromatase Inhibitors. Of the 3425 patients enrolled in this study, 3420 patients were randomly assigned 1:1 to receive PROLIA® 60 mg (N=1711) or matching placebo (N=1709), subcutaneously every 6 months, during Aromatase Inhibitor therapy. Majority of the patients participating in this study had breast cancer with good prognosis and only 25% of the patients required adjuvant chemotherapy. Patient received a median of 7 doses of PROLIA®. The Primary endpoint was the time to first clinical fracture after randomization. The Secondary endpoint was Disease Free Survival (defined as time from randomization to first evidence of local or distant metastasis, contralateral breast cancer, secondary carcinoma, or death from any cause) in the intention-to-treat population. The Primary endpoint of the ABCSG-18 trial was met (The Lancet 2015;386:433-443) and the use of PROLIA® as an adjuvant to Aromatase Inhibitor therapy significantly delayed time to first clinical fracture, compared to Placebo (HR=0.50; P<0.0001). The authors in publication reported the Secondary endpoint of Disease Free Survival (DFS) outcomes from this study.
After a median follow-up of 73 months, there was a significant improvement in the Disease Free Survival among patients in the PROLIA® group compared to the placebo group (HR=0•82; P=0.026). In the PROLIA® group, the DFS was 89.2% at 5 years and 80.6% at 8 years of follow-up, compared with 87.3% at 5 years and 77.5% at 8 years in the placebo group. The total number of adverse events was similar in the PROLIA® and placebo groups and there were no reported cases of osteonecrosis of the jaw bone or atypical femoral fractures.
It was concluded that PROLIA® constitutes an effective and safe adjuvant treatment for patients with postmenopausal hormone receptor-positive early breast cancer receiving Aromatase Inhibitor therapy, with a Disease Free Survival benefit similar to bisphophonates, but with a better toxicity profile and convenient subcutaneous injections twice yearly. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Gnant M, Pfeiler G, Steger GG, et al. Lancet Oncol 2019;20:339-351

