SUMMARY: The American Cancer Society estimates that in the US, about 4000-5000 cases of Gastro Intestinal Stromal Tumors (GISTs) are diagnosed each year. GI Stromal Tumor (GIST) is one of the most common types of Soft Tissue Sarcoma and can develop anywhere along the GI tract, but are primarily found in the stomach. GISTs originate from the interstitial cells of Cajal or related stem cells and are associated with activating mutations in KIT or PDGFRA (Platelet-Derived Growth Factor Receptor-A). These two mutations are mutually exclusive and are important in the molecular pathogenesis of these tumors. Treatment of patients with advanced or metastatic GIST with Tyrosine Kinase Inhibitor GLEEVEC® (Imatinib) achieves high Objective Response and diseases stabilization rates. Patients with KIT exon 9 mutation have a poor prognosis compared to those with KIT exon 11 mutation and benefit from a higher dose of GLEEVEC® (800 mg daily). It should also be noted that patients with PDGFRA D842V mutation are GLEEVEC® resistant. Approximately two thirds of the patients with GISTs are cured with surgery but recurrences are frequent and this risk of relapse is dependent on the tumor size, mitotic rate and primary tumor site. The risk stratification of GISTs by Joensuu, unlike the NIH criteria, takes into account primary tumor site and tumor rupture as well, which can influence outcomes.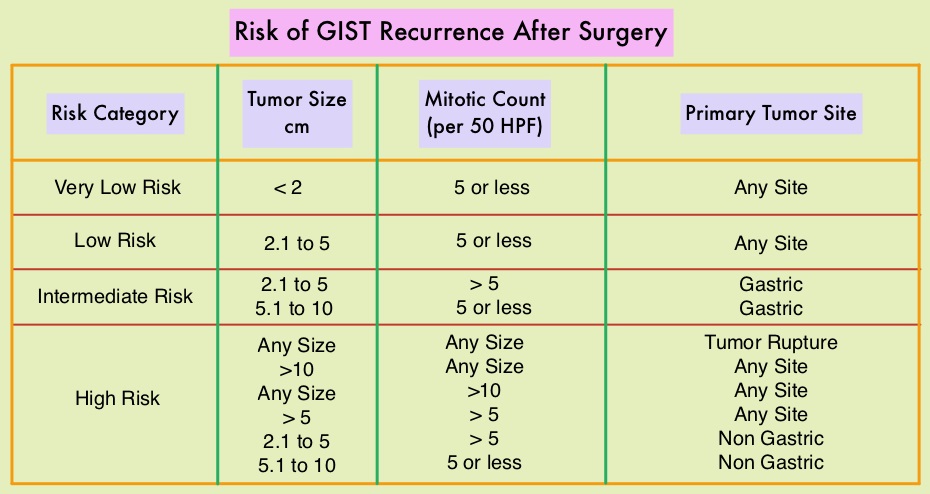
Adjuvant therapy with three years of GLEEVEC®, following curative surgery of high risk GISTs, is recommended and has been shown to improve Recurrence Free Survival (RFS). However, whether adjuvant GLEEVEC® improves overall survival has remained unclear. To address this further the authors in this publication performed a second planned analysis of the SSGXVIII/AIO trial after a longer follow up. In this open label study, 400 patients following surgery were randomly assigned to receive adjuvant GLEEVEC® for either 12 months (N=200) or for 36 months (N=200). Eligible patients had completely resected, KIT-positive GIST, with high risk features, per the modified National Institutes of Health criteria. The median age was 61 years and the primary objective was Recurrence Free Survival (RFS), and the secondary objectives included Overall Survival and Safety.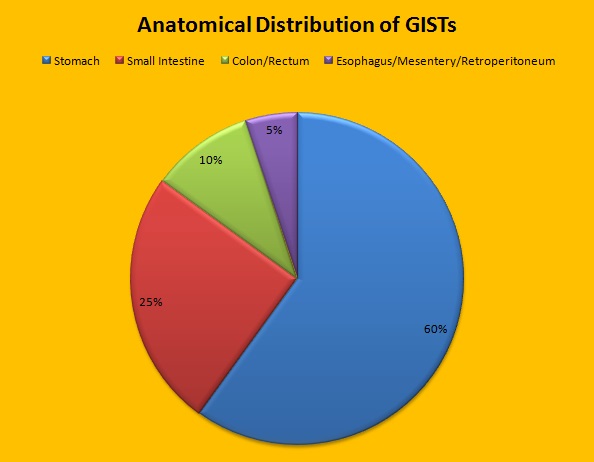
The second planned analysis was done 3 years after the first analysis of the trial and was intended to compare Overall Survival between the treatment groups, after additional follow up of these patients. The median follow up was 90 months. It was noted that the patients who were treated with 36 months of adjuvant GLEEVEC® had a longer RFS than those who were treated for 12 months (5-year RFS was 71.1% versus 52.3%, HR=0.60; P<0.001). The Overall Survival (OS) also favored the 36 month treatment group compared with the 12 month treatment group (5-year OS was 91.9% versus 85.3%, HR=0.60; P=0.036).
The authors concluded that 3 years of adjuvant GLEEVEC® therapy results in longer Overall Survival than 1 year of adjuvant GLEEVEC®, with a superior 5-year survival rate, in patients with high-risk GIST. Trials are underway evaluating the benefit of adjuvant GLEEVEC® for longer than 3 years. Adjuvant Imatinib for High-Risk GI Stromal Tumor: Analysis of a Randomized Trial. Joensuu H, Eriksson M, Hall KS, et al. J Clin Oncol 2016;34:244-250

