SUMMARY: Prostate cancer is the most common cancer in American men excluding skin cancer and 1 in 7 men will be diagnosed with prostate cancer during their lifetime. It is estimated that in the United States, over 230,000 new cases of prostate cancer will be diagnosed in 2014 and close to 30,000 men will die of the disease. Prostate cancer is driven by Androgen Receptor (AR) and its signaling pathways. Initial treatment strategies for patients with metastatic prostate cancer include lowering the levels of circulating androgens with medical or surgical castration or blocking the binding of androgens to the androgen receptor. 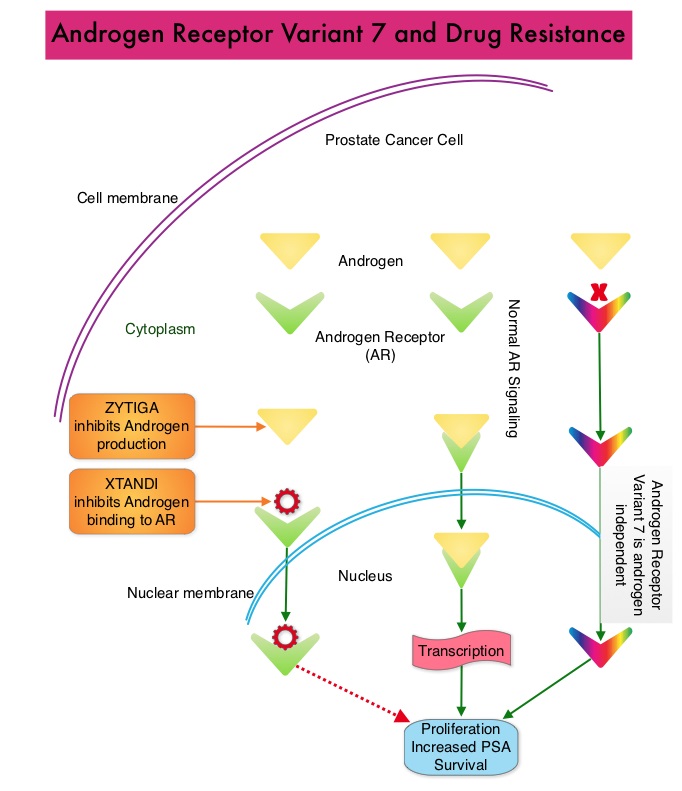 Upon progression {described as Castrate Resistant Prostate Cancer (CRPC), as these tumors are not androgen independent and continue to rely on Androgen Receptor signaling} two agents are presently available for metastatic CRPC. They include ZYTIGA® (Abiraterone) and XTANDI® (Enzalutamide). Both these agents have been shown to improve survival in metastatic CRPC. ZYTIGA® inhibits CYP 17A1 enzyme and depletes adrenal and intratumoral androgens, thereby impairing AR signaling. XTANDI® competes with Testosterone and Dihydrotestosterone and avidly binds to the Androgen Receptor, thereby inhibiting AR signaling and in addition inhibits translocation of the AR into the nucleus and thus inhibits the transcriptional activities of the AR. About 20-40% of the patients do not respond to these newer agents and even those who respond will invariably develop resistance to these drugs. This again has been attributed to persistent AR signaling by variant forms of Androgen Receptor, generated through somatic mutation or aberrant RNA splicing. Androgen Receptor Variant AR-V7 can be detected in the circulating tumor cells. AR-V7 does not have the domain to bind androgens and may be associated with resistance to XTANDI®. Further AR-V7 is constitutively active and can independently activate transcription factors and therefore is not effected by androgen depleting agents including ZYTIGA®. With this background, the authors hypothesized that detection of Androgen Receptor variant AR-V7 in circulating tumor cells from men with metastatic prostate cancer would be associated with resistance to both ZYTIGA® and XTANDI®. In this prospective study which enrolled patients with Castrate Resistant Prostate Cancer (CRPC), 31 patients received treatment with ZYTIGA® and 31 patients received treatment with XTANDI®. Levels of AR-V7 in circulating tumor cells of these patients were analyzed using quantitative Reverse Transcriptase – Polymerase Chain Reaction assay. The primary endpoint was association between AR-V7 status (positive versus negative) and Prostate Specific Antigen (PSA) response rates and secondary endpoints included freedom from PSA progression (PSA Progression Free Survival), clinical or radiographic Progression Free Survival, and Overall Survival. The authors noted that patients with detectable AR-V7 in circulating tumor cells had no response to ZYTIGA® or XTANDI® as measured by serum PSA level reduction of 50% or more and also had a shorter Progression Free Survival and Overall Survival. Also of interest, the prevalence of detectable AR-V7 in circulating tumor cells before treatment with ZYTIGA® and XTANDI® was 9-15% whereas it increased to approximately 50% after disease progressed during treatment with either of these two drugs. This suggested a common mechanism of resistance to both drugs. The authors concluded that detection of AR-V7 in circulating tumor cells from patients with Castration Resistant Prostate Cancer, may be associated with resistance to ZYTIGA® and XTANDI® and if further validated, could be used as a biomarker. Antonarakis ES, Lu C, Wang H, et al. N Engl J Med 2014; 371:1028-1038
Upon progression {described as Castrate Resistant Prostate Cancer (CRPC), as these tumors are not androgen independent and continue to rely on Androgen Receptor signaling} two agents are presently available for metastatic CRPC. They include ZYTIGA® (Abiraterone) and XTANDI® (Enzalutamide). Both these agents have been shown to improve survival in metastatic CRPC. ZYTIGA® inhibits CYP 17A1 enzyme and depletes adrenal and intratumoral androgens, thereby impairing AR signaling. XTANDI® competes with Testosterone and Dihydrotestosterone and avidly binds to the Androgen Receptor, thereby inhibiting AR signaling and in addition inhibits translocation of the AR into the nucleus and thus inhibits the transcriptional activities of the AR. About 20-40% of the patients do not respond to these newer agents and even those who respond will invariably develop resistance to these drugs. This again has been attributed to persistent AR signaling by variant forms of Androgen Receptor, generated through somatic mutation or aberrant RNA splicing. Androgen Receptor Variant AR-V7 can be detected in the circulating tumor cells. AR-V7 does not have the domain to bind androgens and may be associated with resistance to XTANDI®. Further AR-V7 is constitutively active and can independently activate transcription factors and therefore is not effected by androgen depleting agents including ZYTIGA®. With this background, the authors hypothesized that detection of Androgen Receptor variant AR-V7 in circulating tumor cells from men with metastatic prostate cancer would be associated with resistance to both ZYTIGA® and XTANDI®. In this prospective study which enrolled patients with Castrate Resistant Prostate Cancer (CRPC), 31 patients received treatment with ZYTIGA® and 31 patients received treatment with XTANDI®. Levels of AR-V7 in circulating tumor cells of these patients were analyzed using quantitative Reverse Transcriptase – Polymerase Chain Reaction assay. The primary endpoint was association between AR-V7 status (positive versus negative) and Prostate Specific Antigen (PSA) response rates and secondary endpoints included freedom from PSA progression (PSA Progression Free Survival), clinical or radiographic Progression Free Survival, and Overall Survival. The authors noted that patients with detectable AR-V7 in circulating tumor cells had no response to ZYTIGA® or XTANDI® as measured by serum PSA level reduction of 50% or more and also had a shorter Progression Free Survival and Overall Survival. Also of interest, the prevalence of detectable AR-V7 in circulating tumor cells before treatment with ZYTIGA® and XTANDI® was 9-15% whereas it increased to approximately 50% after disease progressed during treatment with either of these two drugs. This suggested a common mechanism of resistance to both drugs. The authors concluded that detection of AR-V7 in circulating tumor cells from patients with Castration Resistant Prostate Cancer, may be associated with resistance to ZYTIGA® and XTANDI® and if further validated, could be used as a biomarker. Antonarakis ES, Lu C, Wang H, et al. N Engl J Med 2014; 371:1028-1038

