SUMMARY: The American Cancer Society estimates that 73,750 new cases of kidney and renal pelvis cancers will be diagnosed in the United States in 2020 and about 14,830 people will die from the disease. Renal Cell Carcinoma (RCC) is by far the most common type of kidney cancer and is about twice as common in men as in women. Modifiable risk factors include smoking, obesity, workplace exposure to certain substances and high blood pressure. The five year survival of patients with advanced RCC is less than 10% and there is significant unmet need for improved therapies for this disease. SUTENT® (Sunitinib) is a MultiKinase Inhibitor (MKI) which simultaneously targets the tumor cell wall, vascular endothelial cell wall as well as the pericyte/fibroblast/vascular/smooth vessel cell wall and is capable of specifically binding to tyrosine kinases, inhibiting the earlier signaling events and thereby inhibits phosphorylation of VEGF receptor, PDGF receptor, FLT-3 and c-KIT. SUTENT® is the standard first-line intervention for treatment naïve patients with advanced RCC. In a large, multi-center, randomized, Phase III study, the median Progression Free Survival (PFS) with SUTENT® was 9.5 months, the Objective Response Rate (ORR) was 25%, and the median Overall Survival was 29.3 months, when compared with Interferon Alfa, in patients with treatment-naïve Renal Cell Carcinoma. This was however associated with a high rate of hematological toxicities.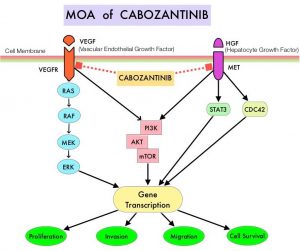
The FDA in 2018, approved combination immunotherapy, OPDIVO® (Nivolumab) plus YERVOY® (Ipilimumab), for the treatment of intermediate or poor-risk, previously untreated advanced Renal Cell Carcinoma (RCC), based on significantly higher Overall Survival (OS) and Objective Response Rates (ORR), compared with SUTENT® (CheckMate 214). Subsequently, two studies, a combination of BAVENCIO® (Avelumab) and INLYTA® (Axitinib) – JAVELIN Renal 101, and KEYTRUDA® (Pembrolizumab) and INLYTA® (KEYNOTE-426), demonstrated superior OS, compared to SUTENT®, and for the first time set the stage for the use of a combination of checkpoint inhibitor and targeted therapy in this patient population.
OPDIVO®, an anti-PD-1 checkpoint inhibitor and CABOMETYX® (Cabozantinib), a small-molecule Tyrosine Kinase Inhibitor, are both approved as single agents, for the second-line treatment of Renal Cell Carcinoma. The rationale for combining these two agents is that OPDIVO® unleashes the immune system and restores antitumor immune response, whereas CABOMETYX® has both antiangiogenic and immunomodulatory properties and may counteract tumor-induced immunosuppression.
CheckMate 9ER study is a multinational, randomized, Phase III trial, in which a combination of OPDIVO® plus CABOMETYX® was compared with single agent SUTENT®, in treatment naïve patients with advanced clear cell Renal Cell Carcinoma. This study included 651 treatment naïve patients with advanced Renal Cell Carcinoma with a clear cell component, who were randomly assigned in a 1:1 ratio to receive OPDIVO® 240 mg IV every 2 weeks along with CABOMETYX® 40 mg orally daily (N=323) or SUTENT® 50 mg orally daily in 4-week-on, 2-week-off cycles (N=328). Treatment was continued until disease progression or unacceptable toxicity. Patients with any IMDC (International Metastatic RCC Database Consortium) risk score were included. Patients with sarcomatoid tumor features were allowed. Patients were stratified by IMDC risk score and tumor PD-L1 expression. The median patient age was 62 years, 58% of patients were in the IMDC intermediate risk category and 75% of patients had tumor PD-L1 expression of less than 1%. The Primary endpoint was Progression Free Survival (PFS) and Secondary endpoints included Overall Survival (OS), Objective Response Rate (ORR) and safety.
At a median follow up of 18.1 months, the median PFS was 16.6 months with OPDIVO® plus CABOMETYX® combination versus 8.3 months with single agent SUTENT® (HR=0.51; P<0.0001), suggesting a doubling of PFS, with a 49% reduction in the risk of disease progression or death. The median Overall Survival, a secondary endpoint, was not reached in either treatment group, but at this first analysis, patients randomized to the OPDIVO® plus CABOMETYX® combination had significantly longer OS, than those receiving SUTENT® (median Not Reached; HR=0.60; P=0.001), suggesting a 40% reduction in the risk of death. These benefits were seen consistently across pre-specified subgroups defined according to IMDC risk categories and PD-L1 expression. The Objective Response Rate (ORR) was also significantly higher and doubled among patients receiving the OPDIVO® plus CABOMETYX® combination, compared to those receiving SUTENT® (55.7% versus 27.1%, P<0.0001). Complete response rates were also higher among those receiving the OPDIVO® plus CABOMETYX® combination (8.0% versus 4.6%), with a shorter median time to response, and longer duration of response. Grade 3 or more Adverse Events were higher among those receiving OPDIVO® plus CABOMETYX® combination, compared to those receiving SUTENT® (60.6% versus 50.9%).
It was concluded that a combination of OPDIVO® plus CABOMETYX® demonstrated superior Progression Free Survival, Overall Survival and Overall Response Rate, compared to SUTENT®, in treatment naïve patients with advanced Renal Cell Carcinoma, and provides a new treatment option for this patient group.
Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: first results from the randomized phase 3 CheckMate 9ER trial. Choueiri TK, Powles T, Burotto M, et al. Ann Oncol. 2020;31(4). Abstract 696O.

